
The intriguing, upcoming field of quantum biology by Olivier Loose.
Scientific inquiry can be a lesson in humility. Astronomer Nicolaus Copernicus taught us this in the sixteenth century when submitting the hypothesis that the Sun is sitting at the centre of the Solar System, not the Earth.
Although we are limited by our own cognitive abilities, such humility can nevertheless open doors towards a broader understanding of the intrinsic dynamics of life.
Can we learn about biological processes through quantum physics, even though such new insights may fly in the face of our present knowledge?
The Apparent Conundrum
Classical versus Quantum Mechanics
Classical mechanics – the physics developed by Galileo Galilei, Isaac Newton and others – explains the movement of objects in the macroscopic world. Flinging your phone high into the sky and seeing it coming crashing down on the floor is an example of a trajectory entirely described by these classical laws. If you have some initial parameters, such as starting velocity and the angle under which your phone takes off, you can predict beforehand where your phone will land and with what speed. In other words, the world of large objects is deterministic.
As we zoom in to the level of individual particles, scientists, including Niels Bohr, Werner Heisenberg and Erwin Schrödinger, have come to observe that the classical laws did not hold. This is the realm of quantum mechanics, which is inherently probabilistic. Heisenberg reflected this characteristic in his uncertainty principle, which tells us that it is impossible to know simultaneously where a particle is situated in space and what its velocity is. With the help of a mathematical concept, i.e. the probability wave function, we can work out the chance that a particle will be at a certain location. This uncertainty stems from the fact that a particle also displays wave-like features in the microscopic terrain of quantum physics.
Quantum Vocabulary
A couple of other quantum idiosyncrasies that are pertinent to this article are quantum coherence, quantum entanglement and quantum tunneling.
Quantum coherence refers to the phenomenon whereby two or more particles of a system vibrate in phase with each other. In such coherent system, the particles find themselves in all their possible states at the same time, i.e. superposition, because of their wave-like essence. Once a measurement on the system is performed or the system comes into contact with its surroundings, one particular state is ‘chosen’, and the coherence is broken. At this moment, the system has effectively transitioned from quantum to classical behaviour.
Dephasing alludes to this progression from quantum to classical behaviour, and quantum beats are a way to quantify quantum coherent aspects of a system.
Quantum entanglement is the interdependent relationship between two or more particles. That is, a change in the properties of one particle instantaneously modifies the property of the other particle(s), regardless of the distance between them. It is important to note that the totality of the entangled particles acts as a single, coherent system.
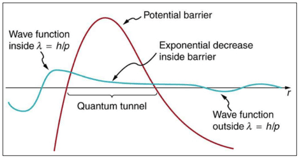
Quantum tunneling occurs when a particle in a coherent system moves through an energy barrier that is otherwise forbidden to transverse in the classical, macroscopic domain. This happens as a result of the wave character of a particle in quantum physics.
The World Inside Out
It is far from evident to experimentally establish quantum coherence. In meticulously manipulated lab settings isolated from its environment, the system usually has to be cooled down to temperatures close to absolute zero whereby lasers induce coherence.
Biological life is not an isolated environment though. And temperatures are well above absolute zero. What is more, chemical reactions have so far done a great job in accounting for the inherent dynamics of our entire ecosystem, without banking too much on the quirks of quantum physics.
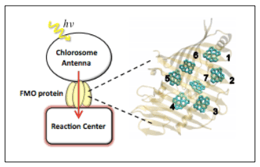
And yet, against all odds, the field of quantum biology suggests that quantum mechanical processes could persist in the turbulent and warm atmosphere of life. For the case of photosynthesis, the first experimental evidence hereof came in 2007 by Gregory Engel et al., when studying the Fenna-Matthews-Olson (FMO) complex in green sulphur bacteria. FMO is a light-harvesting protein complex that regulates the transfer of excited energy to the reaction centre in these bacteria. These authors reach the conclusion that the “wavelike characteristic of the energy transfer within the photosynthetic complex can explain its extreme efficiency”.
Thereafter, the field has been flourishing, and scientists continue to discover the presence of quantum events in different natural milieus. As a case in point, Armin Shayeghi et al. have detected for the first time how a natural antibiotic behaves like a quantum wave. And Junxu Li and Sabre Kais have recently documented how quantum entanglement can be assessed in chemical reactions.
Indeed, quantum biology has been delving into various biological phenomena in which quantum effects may play a part. They include, among others, Brownian motors in cellular processes, magnetoreception, cellular respiration, enzyme-catalyzed reactions, visual phototransduction, olfaction, deoxyribonucleic acid (DNA) mutation and photosynthesis.
In this article, I look under the hood of two particular biological mechanisms: photosynthesis and DNA mutation.
Photosynthesis
Plants and some organisms dispose of the ability to apply sunlight for the production of life-sustaining resources. Nature seems capable to execute that function in an incredibly efficient manner, which leaves researchers, to this day, debating about its underlying causes and modus operandi.
One line of scientific thought, i.e. quantum biology, is figuring out whether quantum physics can provide the missing piece of the puzzle in this quest for clarification. Are the molecular vibrations telling us the whole story? Or are we only able to complete the circle by incorporating quantum mechanics?
Time for some studious reflection.
Let There Be Life
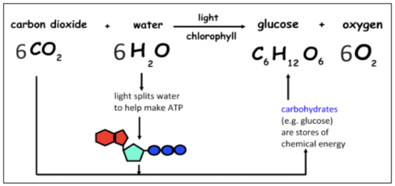
Due to the work of Jan van Helmont, Joseph Priestley and others, we now know that photosynthesis is a natural process whereby carbon dioxide (CO2) and water (H20) synthesize into glucose (C6H12O6) with the support of sunlight. Oxygen (O2) is hereby delivered as a by-product. The key attribute of the photosynthetic mechanism is the conversion of solar energy into chemical energy, and it can be carried out by phytoplankton, cyanobacteria and plants.
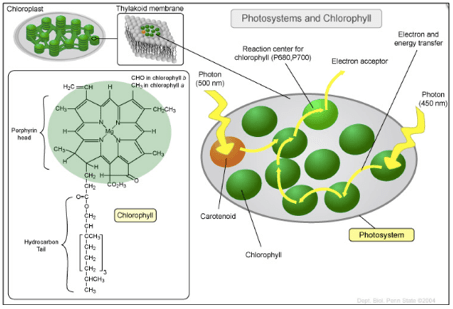
Within plant leaf cells, photosynthesis arises in organelles called chloroplasts (see Fig. 3). They contain photosystems where light-harvesting takes place. Light-harvesting encompasses the capture of solar energy as well as its transfer to reaction centres.
The photosystem is riddled with antenna pigment molecules, i.e. chromophores, that receive sunlight in the form of photons. Upon reception of a photon, these chromophores, such as chlorophyll and carotenoid, expel an electron. Afterwards, by the agency of photoinduced electron transfer reactions, that electron finds its way to a chlorophyll molecule within the reaction centre. Eventually, it ends up in an electron acceptor, which can be thought of as the exit of the photosystem.
The most crucial part of photosynthesis transpires in the reaction centre where the solar energy is transposed into a charge separation, i.e. chemical energy.
At the same time, an electron transport chain is responsible for the creation of the energy-storing molecules adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide phosphate (NADPH). Within the chloroplast and outside of the photosystem, both ATP and NADPH are subsequently transformed into stores of chemical energy carbohydrates, e.g. glucose.
Light-harvesting is “almost 100% efficient”, say Graham Fleming and Gregory Scholes. But given our warm and chaotic natural environment, the question is how such high productiveness of energy transfer in photosynthesis is cultivated.
The conundrum comes down to whether this efficiency is due to a quantum signature within the molecular vibrations.
A Classical View
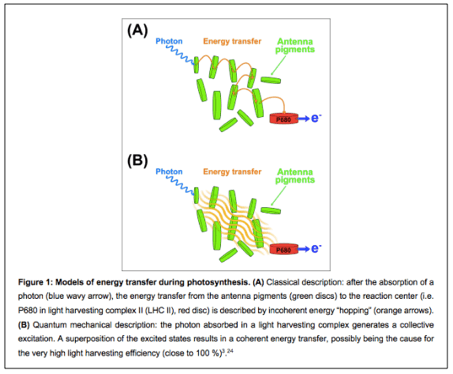
Since a photon-receiving chromophore finds itself in an excited state, its molecular vibrational mode can be transferred to a neighbouring, less excited chromophore with the assistance of electromagnetic interactions. This mechanism is referred to as ‘electron hopping’, and the charge cascades from higher to lower molecular energy levels.
Such classical explanations, which are underpinned by theories like generalized Marcus theory, Förster theory and incoherent Redfield theory, hypothesize that the partial orbital overlap of the molecules allows for the electron transfer as a consequence of energetic resonance.
The route from the chromophore to the reaction centre is hereby assumed to be completely random. Some paths are prioritized over others, depending on whether incoming sunlight is being usefully utilized. Surely, even if light-harvesting is nearly perfectly efficient, there still exists some energy waste along these pathways, disguised as dissipated heat or fluorescence.
Despite the fact that the field of quantum biology is gaining traction, many scientists remain skeptical about long-lived quantum coherence in living organisms.
As an illustrative example, the research of Vivek Tiwari et al. asserts that molecular vibrations account for the highly productive energy transfer rather than quantum coherence. Margherita Maiuri et al. and Jacob Dean et al. corroborate such findings during their examination of green sulphur bacteria and cryptophyte algae, respectively.
And also Duan Hong-Guang et al. reason along the same lines. In fact, they explicitly mention that “contributions of quantum coherence to biological functionality under ambient conditions in natural light-harvesting units is extremely unlikely”. In addition, they report that these results are probably general.
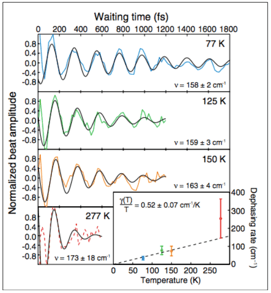
In the context of green sulphur bacteria, Alexei Halpin et al. indeed point out that the established coherence in the FMO complex breaks down too rapidly to have any relevant importance for the energy transfer process. Moreover, Erling Thyrhaug et al. maintain that these coherences are inherently vibrational. In 2019, Shawn Irgen-Gioro et al. drew similar inferences regarding the time scale for coherence for both green sulphur and purple nonsulphur bacteria.
With reference to the photosynthetic protein PC645 in cryptophyte algae, Samuel Blau et al. are inclined to endorse a classical explanation for this formidable performance in energy transfer. In particular, they avouch that “The incoherent vibronic mechanism assigned here to PC645 is far more robust to imperfections than its coherent counterpart”.
Recently, Chanelle Jumper et al. sketched out the state of play of the academic debate on coherent and incoherent transfer dynamics in green sulphur bacteria and algae. Their research favours a vibronic coupling framework, which “does not involve a coherent energy transfer mechanism”. “Instead”, they continue, “it is an elaboration of Förster theory.”
A Quantum Narrative
Yet, in view of a virtually perfect efficiency, an increasing number of scientists plead that quantum coherence is written all over these molecular vibrations.
From a quantum perspective, an electron acts, besides as a particle, like a wave. Therefore, it would ‘test’ beforehand all the available routes to the reaction centre, for as long as the quantum coherence endures. Ultimately, it ‘selects’ the one with the least amount of energy loss.
Edward O’Reilly and Alexandra Olaya-Castro concur with such reasoning. They reveal that “coherent vibrational motions that do not relax quickly and whose fluctuations cannot be described classically may be seen as an internal quantum mechanism controlling energy distribution and storage.” Dugan Hayes et al., for instance, come to analogous conclusions by examining small molecules called synthetic heterodimers. However, they also state that, arguably, “Coherent effects may increase energy transfer efficiency relative to strictly incoherent transfer mechanisms.”
Surprisingly, quantum coherence can be sustained in a natural setting, not only during a deeply cooled, artificial lab experiment. As a matter of fact, Francesca Fassioli et al. point to “new evidence indicat[ing] that electronic coherence can survive in the biological environment for weaker electronic coupling than previously thought.” Actually, quantum coherence has been identified in chlorophyll molecules of green sulphur bacteria, marine algae and plants.
When scrutinizing green plants, Elisabet Romero et al. disclose data that unveils “the strong correlation between the degree of electronic coherence and efficient and ultrafast charge separation.” That is to say, quantum coherence may enhance the energy transport towards the reaction centre and the light-harvesting machinery in general.
In the case of green sulphur bacteria, Davinder Singh and Shubhrangshu Dasgupta observe that “the coherence between the different [bacteriochlorophyll-a] sites is an essential ingredient for the success of [excitation energy transfer (EET)].” Furthermore, they proclaim that, notwithstanding “the presence of pigment-pigment couplings, the absence of coherence prohibits the EET.”
Turning our attention to cryptophyte marine algae, Elisabetta Collini et al. similarly remark that, at ambient temperatures, “distant molecules within the photosynthetic proteins are ‘wired’ together by quantum coherence for more efficient light-harvesting”.
Somewhat counterintuitively, Gabriela Schlau-Cohen et al. ascertain that even the immediate surroundings are conducive to quantum coherence during the energy transfer in green plants. More often than not, contact with the environment entails the end of coherence. Intriguingly, Masoud Mohseni et al. find comparable indications in their exploration of the FMO complex in green sulphur bacteria.
To take it a step further, Mohan Sarovar et al. demonstrate that not only quantum coherence but also quantum entanglement appears to manage the energy transfer in the FMO complex. Contrary to standard quantum experiments, temperature did not seem to notably influence the strength of entanglement in their research. Interestingly, the data analysed by Fernando Galve et al. suggests matching interpretations when inspecting systems out of thermal equilibrium.
Quantum entanglement in living organisms remains an active field of scientific inquiry. More recently, Chiara Marletto et al. have probed green sulphur bacteria and confirmed the involvement of quantum entanglement in their photosynthetic elements.
Wrong Question, Perhaps?
What if the original question whether the energy transfer efficiency in photosynthesis calls for a quantum faculty was not framed in a particularly constructive way?
Maybe we will reach novel insights, only if we go beyond the apparent conundrum of adopting either a quantum or classical approach. In fact, Akihito Ishizaki and Graham Fleming treat the issue of energy transfer in a nondualistic fashion. In concrete terms, they designed a mathematical structure that “describe[s] quantum coherent wavelike motion and incoherent hopping in a unified manner.”
Is such conceptual unification also being considered for another biological process, namely that of DNA mutation?
DNA Mutation
Every so often, the vibrant atmosphere within our microscopic DNA ecosystem leads to a slight rearrangement of biomolecules, which can bring about mutations in our genes. For this reason, we may develop genetic disorders, including colon cancer, Klinefelter Syndrome, Huntington Disease or Cri du Chat Syndrome.
Scientists dig into the root causes of these shifts in our fundamental DNA material to better comprehend how these maladies form. One cross-disciplinary field of research, i.e. quantum biology, oversees whether quantum mechanics has anything to do with these molecular permutations.
Let us have a look.
What Are We Fundamentally Made Of?
DNA is a molecule that retains genetic information. It is vital for passing on the specific traits of a species to their next generation. The DNA’s foundational building blocks consist of four chemical bases: adenine (A), thymine (T), cytosine (C) and guanine (G). A DNA base pair is the coupling of either A and T or C and G. Combining a base, a sugar and a phosphate molecule gives us a nucleotide. Sequencing a number of nucleotides results in a gene, which gives expression to either ribonucleic acid (RNA) or a protein. A long strand of DNA that contains many genes constitutes a chromosome. According to the Watson-Crick DNA model, hydrogen bonds between the base pairs are holding the entire DNA double helix architecture together.
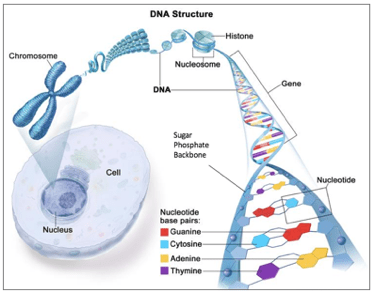
DNA replicates itself at an on-going basis to ensure the production of new cells. During the replication procedure, the double helix is broken up, so that the two individual DNA strands can be copied. In the final stage, the stands are put back together.
DNA mutation refers to any modification in the order or the composition of the nucleotides. It can be caused by either an external factor, e.g. ultraviolet radiation, or a spontaneous error at the time of DNA reproduction. There exist different kinds of mutation, including missense mutation, nonsense mutation, insertion, deletion, duplication, frameshift mutation and repeat expansion. If only a single nucleotide is affected, we speak of a point mutation. Mutations can facilitate the introduction of new species by means of genetic variation. Without DNA mutations, we would not witness evolution in living organisms at all.
Sometimes, the proton in the hydrogen bond can spontaneously swap position – this chemical reaction is called tautomerism. A point mutation can then ensue, only if the DNA replication scheme occurs exactly when the nucleotide base transfers to its rare tautomeric form – James Watson and Francis Crick first proposed this idea in 1953. The outcome is the formation of an incorrect DNA base pair, for example a T-G pair (see Fig. 9). There are also other types of spontaneous mutations, which we will not deal with in this article.
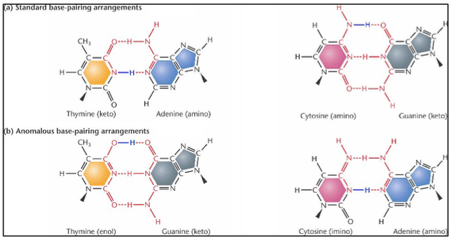
What scientists have been discussing in recent decades translates into whether such tautomeric shifts are ascribed to either the classical ‘proton hopping’ or quantum tunneling.
A Classical Perspective
DNA mutations are considered to be random – mind you, natural selection is not. And, in the context of tautomerism, they are propelled by ‘proton hopping’.
Classically, such hopping sets in as soon as the proton gains a minimum amount of activation energy to overcome a potential energy barrier. At that point, the proton switches position in the hydrogen bond to form a tautomer (see Fig. 9).
With respect to tautomerization of the G-C base pair, Nigora Turaeva and Victoria Brown-Kennerly, for instance, review the likelihood of spontaneous mutations by virtue of these tautomers, leaning on the classical two-dimensional Marcus theory.
As regards the A-T pair, Adam Godbeer et al. actually calculated the possibility of quantum tunneling. They affirm that this quantum phenomenon is not likely “to be a significant mechanism for the creation of adenine-thymine tautomers within DNA”. Moreover, Ashkan Shekaari and Mahmoud Jafari draw similar conclusions for the same base pair.
In response to the paper of Adam Godbeer and colleagues, Ol’ha Brovarets and Dmytro Hovorun take an even more critical stance. In their assessment, they infer that “Tunneling of the protons […] in the A∙T(WC) base pair should not be observed at all under the condition of adequate parameterization of the model”.
However, there are researchers that come to different results.
A Quantum Explanation
In the hydrogen bond between two nucleotide bases, the proton is being pulled forth and back by two lone electrons. From a quantum mechanical perspective, the protons are in superposition. At the moment of DNA replication, they will have to ‘choose’ to attain either a normal or a tautomeric form of base pairs.
Because a particle also possesses a wave-like nature, it can enter into classically forbidden zones and travel straight through a potential energy barrier. What is more, given the minimal mass of the hydrogen atom, hydrogen bonds may be more susceptible to quantum events like quantum tunneling.
More generally, Jan Meisner and Johannes Kästner signal a maturing academic acceptance of quantum tunneling in chemical reactions. As a case in point, Massimiliano Di Ventra and Masateru Taniguchi examine the role of tunneling in sequencing in relation to drugs and treatments.
Among proponents of quantum biology, it is acknowledged that quantum tunneling explicates the actual proton transfer in tautomerization. Already back in 1963, Per-Olov Löwdin hinted that quantum tunneling guides the transition from normal to tautomeric DNA base pairs. More recently, Frank Trixler reaffirms that, in fact, “proton tunnelling influences the main function of DNA – the reliable storage of genetic information.”
Fig 10 depicts an example of the probabilities of quantum tunneling of a proton for the tautomeric base pair G-T. Considering that the tautomeric form is less stable than its normal equivalent, the potential energy of the proton follows an asymmetric double-well pattern.
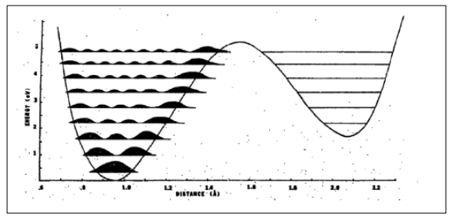
Inasmuch as rare tautomers are not easily experimentally monitored, scientists commonly rely on computational methods to investigate proton relocations within DNA. In one such study, Ruby Srivastava concludes that “There is a least but finite probability for protons to change place within the hydrogen bond due to quantum tunneling, which will alter the genetic code and cause mutations.”
V.L. Golo and Yu.S. Volkov equally link quantum tunneling to DNA mutations. Concretely, they posit that “an action imposed on a set of nucleotide in a region of the molecule might generate mutations in a different region owing to the motion of excitations corresponding to proton tunneling.”To the same extent, J. Luo puts forward that “proton tunnelling across DNA hydrogen bonds may be a mechanism responsible for biological processes such as spontaneous mutation.”
Basing their work on quantum tunneling, E.K. Ivanova et al. specifically look at the prospect of point mutations in function of temperature and DNA replication velocity.
For a more general setting of two identical symmetric hydrogen bonds, Zorka Smedarchina et al. claim that, during the double-proton transfer, quantum entanglement, as well as quantum tunneling, takes place.
Certainly, Onur Pusuluk et al. declare that “tunneling of […] the proton of the H atom can generate useful quantum entanglement in a H-bonded system”. In addition, they submit the stronger hypothesis that “significant amounts of quantum entanglement can be found in the thermal state of hydrogen bond.” Within the specific framework of DNA base pairs, Grant Cooper falls back on the entanglement of protons to portray the conduct of tautomerization.
Video: Quantum mechanics may drive DNA mutations (Duke University).
Wrong Question, Again?
As we have seen, the controversy about quantum biology evolves around the argument whether delicate quantum processes could prevail at the ambient temperatures of biological life. Nonetheless, Wei Fang et al. contend that, unexpectedly, quantum effects “can have a smaller impact on hydrogen bond strengths at cryogenic temperatures than at room temperature.”
Does this mean that quantum mechanics infiltrates into the macroscopic level more extensively than hitherto presumed?
Once again, the question is perhaps not whether quantum physics holds sway over our natural environment. As a matter of fact, also in the context of DNA mutation have several researchers argued away from such dualistic framing.
Concerning hole transfers of electrons in DNA, Yuri Berlin et al., for example, come to the conclusion that both classical hopping and quantum tunneling fulfil a role. They clearly state that “for short AT bridges, hole transfer between two G bases proceeds via quantum mechanical tunneling[, whereas] hopping over long bridges requires thermal activation.”
The Leading Dance of the Small
In our natural habitat, “quantum effects could be more significant than previously anticipated and deserves further study”, insist Wei Fang et al. And some scientists even believe that the next logical question within the field of quantum biology extends to whether these effects could be upscaled from molecules to macroscopic-sized organisms like tardigrades.
Whatever the case may be, quantum biology might well turn out to be a promising candidate that appreciably contributes to improving both the human condition and our planet’s health.
Having a more thorough grip on nature’s trick within the light-harvesting component of photosynthesis could ultimately enhance our solar energy technologies. And obtaining a more comprehensive picture about the relevance of quantum processes in cells may help us to advance closer to a mastery of the development of cancer, myotonic dystrophy, neurofibromatosis or sickle cell anemia.
In the end, Erwin Schrödinger may have been on to something when he said that “we must be prepared to find [living matter] working in a manner that cannot be reduced to the ordinary laws of physics.” (Schrödinger, E. (1967). What Is Life?, Cambridge University Press, pp.76. )
Only when we fully grasp the pertinence of quantum physics to biology, will we also be better geared to appreciate how both the classical and quantum descriptions of life may tango together.
